PHILADELPHIA, PA.- An overactive genetic pathway in muscle stem cells was found to shorten the ends of chromosomes, called telomeres, resulting in DNA damage that impedes the normal healing response, according to a new study by researchers in the Perelman School of Medicine at the
University of Pennsylvania. The researchers believe this finding unveils the body’s origin point for the chronic muscle injuries associated with diseases like Duchenne muscular dystrophy. This work was published in Cell Reports.
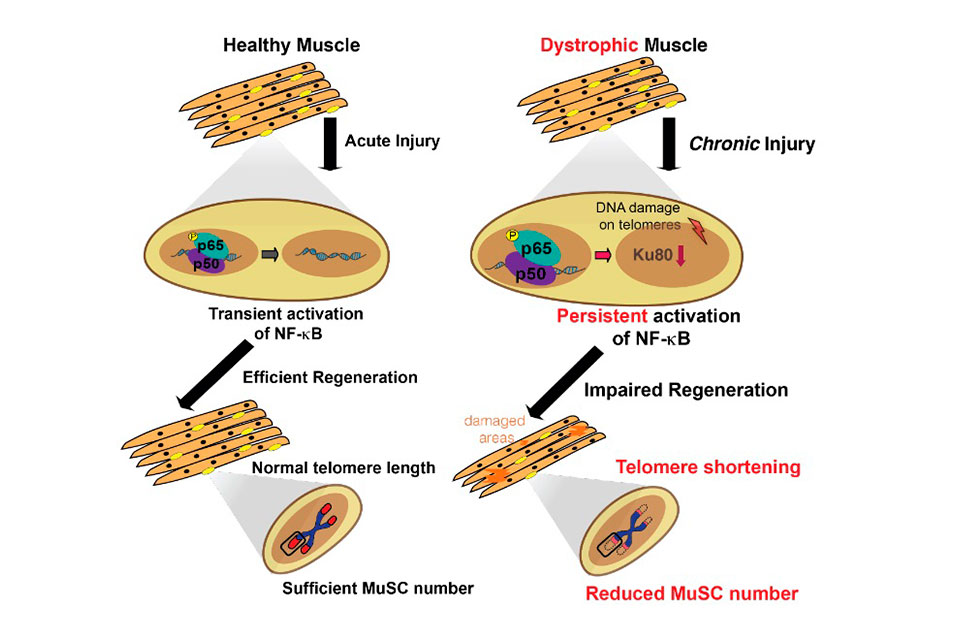
The specific pathway the researchers studied, NF-kB, is tied both to DNA transcription and inflammation response, among other things. It is the first genetic pathway found to directly affect telomere shortening. Shortened telomeres were previously identified as a key feature of patients with Duchenne muscular dystrophy, a severe muscle disease characterized by extensive muscle injury and repair.
The study’s senior author, Foteini Mourkioti, PhD, an assistant professor of Orthopaedic Surgery and Cell and Developmental Biology, as well as the co-Director of the Musculoskeletal Program at the Penn Institute for Regenerative Medicine, said she believes that they have discovered “the tip of a new iceberg for muscle diseases.”
“Our data suggest that maintaining telomere length of muscle stem cells could boost the regenerative ability of the stem cells in disease-related chronic injuries, such as Duchene muscular dystrophy,” Mourkioti said. “It was recently proposed that chronic inflammation can accelerate aging via exacerbation of telomere dysfunction in multiple tissues. Therefore, the molecular mechanism presented here for Duchene muscular dystrophy could also be a more generalized mechanism that lowers regenerative potential in other tissues experiencing widespread chronic inflammation which could also accelerate normal and disease-associated aging.”
Several years ago, Mourkioti’s lab developed a new single-cell imaging technique to closely monitor adult muscle stem cells that were affected by conditions like Duchenne muscular dystrophy. This novel approach has allowed them to observe that dysfunction in these cells progresses over time during the cyclical muscle damage and repair process that occurs within Duchene muscular dystrophy. Telomere shortening was seen to be an important feature that emerges at a very young age in patients, but it wasn’t clear what signaling pathways were driving this finding.
For this study, the researchers, including the study’s lead author, Elisia Tichy, PhD, an instructor in Penn Medicine’s McKay Orthopaedic Research Lab, created assays to examine internal signaling pathways in mice with dystrophy. The researchers noticed a high proportion of NF-kB activation in these cells, which continued at all time points they were observed.
“Chronic injury induced a constant elevated NF-kB response in these cells,” Mourkioti explained.
This activation was found to coincide with the shortening of telomeres, which left the DNA ends exposed and susceptible to damage. Normally, when that occurs muscle stem cells deploy and fix the injured area with no problem. But the researchers were able to show that the persistent high levels of NF-kB reduced a protein called Ku80, which would normally assist other proteins in protecting telomeres. This defect leaves telomeres unprotected, and stem cells are exhausted over time, meaning that muscles couldn’t be repaired.
A lack of muscle repair is the hallmark of Duchenne muscular dystrophy, and the mechanism driving it appears to be unique.
“This new molecular mechanism for telomere shortening is different from what has been shown to occur in the control and proliferation of cancer cells,” Mourkioti said.
The researchers hope to examine this pathway more closely to identify additional avenues that could be better therapeutic targets for diseased muscles and might help in muscle maintenance.
“We hope that building on this work will soon allow us to therapeutically target chronically injured muscles in order to boost their capacity to regenerate and repair themselves,” Mourkioti said.
Other authors on this study include Nuoying Ma, David Sidibe, Emanuele Loro, Jacob Kocan, Delia Chen, Tejvir Khurana, and Paul Hasty.