MONTREAL.- Bacteria can store extra resources for the lean times. It’s a bit like keeping a piggy bank or carrying a backup battery pack. One important reserve is known as cyanophycin granules, which were first noticed by an Italian scientist about 150 years ago. He saw big, dark splotches in the cells of the blue-green algae (cyanobacteria) he was studying without understanding either what they were or their purpose. Since then, scientists have realized that cyanophycin was made of a natural green biopolymer, that bacteria use it as a store of nitrogen and energy, and that it could have many biotechnological applications. They have tried producing large amounts of cyanophycin by putting the enzyme that makes it (known as cyanophycin synthetase) in everything from E. coli to tobacco, but without being able to make enough of it to be very useful.
Now, by combining two cutting-edge techniques, cryo-electron microscopy (at McGill’s Facility for Electron Microscopy Research) and X-ray crystallography,
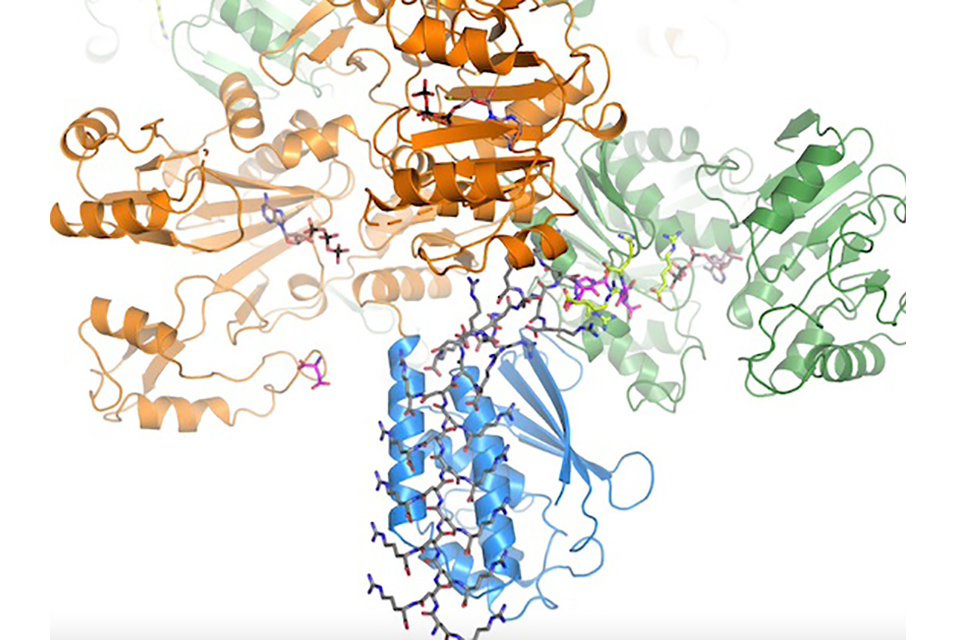
McGill researchers have, for the first time, been able to see the active enzyme in action.
“Until now scientists have been unable to understand the way bacterial cells store nitrogen in cyanophycin, simply because they couldn’t see the enzyme in action,” says Martin Schmeing, a Professor in McGill’s Department of Biochemistry and the senior author on a recent paper on the subject in Nature Chemical Biology. “By stitching 3D images of the enzyme at work into a movie, we were able to see how three different structural units (or domains), came together to create cyanophycin synthetase. It’s a surprising and very elegant example of a natural biomachine.”
The next steps in the research involve looking at the other enzymes used in the complete biosynthesis and degradation cycle of cyanophycin. Once the researchers are able to see them in action, this would potentially give them a complete structural understanding of the processes involved and would allow them to figure out how to turbo-charge cells to make massive quantities of cyanophycin and related polymers for their green polymer biotech applications, such as in biodegradable water softeners and antiscalants or in the creation of heat-sensitive nanovesicles for use in targeted drug delivery.