WASHINGTON, DC.- There’s been a recent push in the U.S. to make naloxone — a fast-acting opioid antidote — available without a prescription. This medication has saved lives, but it’s less effective against powerful synthetic opioids, such as fentanyl. In an interesting twist, researchers are now looking to cannabidiol (CBD), a component of marijuana, as a possible alternative to the popular antidote. Today, a team reports compounds based on CBD that reduce fentanyl binding and boost the effects of naloxone.
The researchers will present their results at the spring meeting of the
American Chemical Society. ACS Spring 2023 is a hybrid meeting being held virtually and in-person March 26–30, and features more than 10,000 presentations on a wide range of science topics.
“Fentanyl-class compounds account for more than 80% of opioid overdose deaths, and these compounds aren’t going anywhere — it’s just too much of an economic temptation for dealers,” says Alex Straiker, Ph.D., the project’s co-principal investigator. “Given that naloxone is the only drug available to reverse overdoses, I think it makes sense to look at alternatives.”
A new option could take one of two forms, according to Michael VanNieuwenhze, Ph.D., the other co-principal investigator for the project.
“Ideally, we would like to discover a more potent replacement for naloxone,” VanNieuwenhze says. “However, finding something that works synergistically with it, reducing the amount needed to treat an overdose, would also be a success.”
Jessica Gudorf, a graduate student in VanNieuwenhze’s group, is presenting the work at the meeting. All of the researchers are at Indiana University Bloomington.
Opioids are a class of compounds that are prescribed to treat pain and are sometimes sold illegally. If taken in excess, the drugs can interfere with breathing, making them potentially lethal. The U.S. Centers for Disease Control and Prevention estimates that more than half a million people died from overdoses involving opioids between 1999 and 2020. That toll continues to climb.
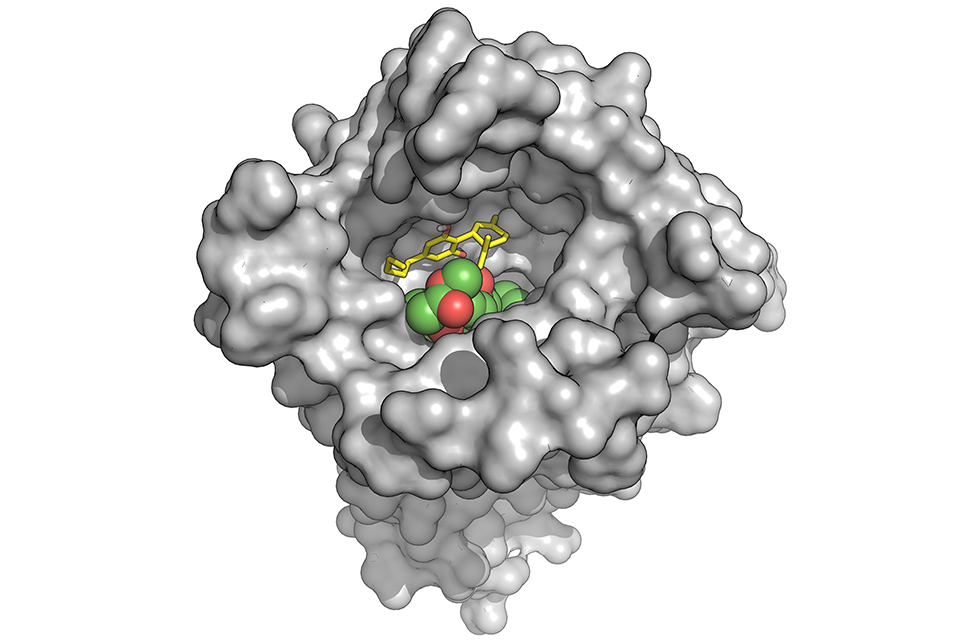
Compared to other compounds in this class, such as heroin or morphine, fentanyl and its other synthetic relatives bind more tightly to opioid receptors in the brain. Naloxone reverses an overdose by competing with the drug molecules for the same binding sites on the receptors. But because fentanyl binds so readily, it has a leg up on naloxone, and growing evidence suggests that reversing these kinds of overdoses may require multiple doses of the antidote.
At this point, researchers have exhaustively studied the strategy naloxone takes, but they have yet to find any way to improve on its performance, Gudorf says. “Our work opens the door to making new blockers that work through a different mechanism,” she explains.
Earlier research suggesting that CBD can interfere with opioid binding inspired the current effort. In research published in 2006, a group based in Germany concluded that CBD hampered opioid binding indirectly, by altering the shape of the receptor. When used with naloxone, they found CBD accelerated the medication’s effect, forcing the receptors to release opioids.
To augment these effects, Gudorf altered CBD’s structure to generate derivatives. Taryn Bosquez-Berger, a graduate student in Straiker’s group, tested these new compounds in cells with a substance called DAMGO, an opioid used only in lab studies. To measure their success, she monitored a molecular signal that diminishes when this type of drug binds. Armed with feedback from these experiments, Gudorf refined the structures she generated.
In the end, they narrowed the field to 15, which they tested at varying concentrations against fentanyl, with and without naloxone. Several derivatives could reduce fentanyl binding even at what Bosquez-Berger described as “incredibly low” concentrations, while also outperforming naloxone’s opioid-blocking performance. Two of these also showed a synergistic effect when combined with the antidote.
The team has since begun testing the most successful derivatives in mice. In these experiments, they are investigating whether these compounds alter behaviors associated with taking fentanyl.
“We hope our approach leads to the birth of new therapeutics, which, in the hands of emergency personnel, could save even more lives,” Bosquez-Berger says.
The researchers acknowledge support and funding from the Indiana University Grand Challenges Program.